Abstract
Introduction. Anti-CD19 chimeric antigen receptor T cells (CART19 or CTL019) have shown impressive clinical activity in B-cell acute lymphoblastic leukemia (B-ALL) and are poised to receive FDA approval. However, some patients relapse after losing CD19 expression. Since CD22 remains highly expressed in relapsed/refractory (r/r) B-ALL even in these patients, anti-CD22 CART (CART22) have been developed. The National Cancer Institute (NCI) reported 4/9 complete remission (CR) in patients receiving CART22, with 100% CR at the highest T cell dose (NCT02315612)(S hah NN, ASH 2016 #650).
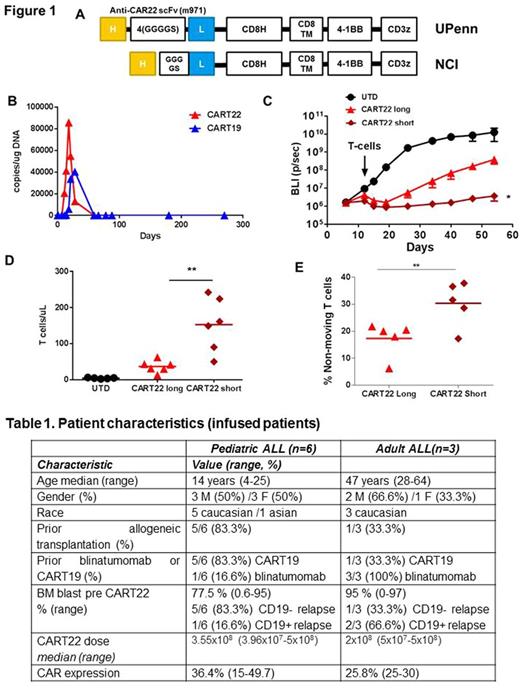
Patients and Methods. We generated a second-generation CAR22 differing from that used by the NCI only by the use of a longer linker [4x(GGGGS); LL vs. 1x(GGGGS); SL] between the light and heavy chains of the scFv (Fig. 1 A). This construct was tested in two pilot clinical trials in adults (NCT02588456)and children with r/r-ALL (NCT02650414). CART22 cells were generated using lentiviral transduction as in our previous studies. The protocol-specified CART22 dose was 2x106-1x107 cells/kg for pediatric patients <50kg and 1-5x108 for pediatric patients ≥50kg and adult patients,. infused after lymphodepleting chemotherapy. Patient characteristics are described in Table 1. For the adult trial, 5 patients were screened, 4 enrolled (1 patient withdrew consent) and 3 infused (1 manufacturing failure). For the pediatric trial, 9 patients were screened, 8 enrolled (1 screen failure) and 6 infused (two patients were not infused for disease progression).
For the preclinical studies, we generated CART22LL and CART22SL and tested them in vivo using xenograft models. NOD-SCID gamma chain deficient (NSG) mice were engrafted with either a luciferase+ standard B-ALL cell line (NALM6) or primary B-ALL cells obtained from a patient relapsing after CART19 (CHP110R). We also used 2-photon imaging to study the in vivo behavior and immune synapse formation and flow cytometry to asses T cell activation.
Results. CART22 cells were successfully manufactured for 10/12 patients. In the adult cohort 3/3 patients developed CRS (gr.1-3) and no neurotoxicity was observed; in the pediatric cohort out of 5 evaluable patients (1 discontinued for lineage switch to AML on pre-infusion marrow), 3/5 developed cytokine-release syndrome (CRS) (all grade 2) and 1 patient had encephalopathy (gr.1). CART22 cells expanded in the PB with median peak of 1977 (18-40314) copies/ug DNA at day 11-18. Interestingly, in an adult patient who had previously received CART19 a second CART19 re-expansion was observed following CART22 expansion (Fig 1 B). At day 28, in the adult cohort the patient who was infused in morphologic CR remained in CR, while the other 2 had no response (NR); in the pediatric cohort 2/5 patients were in CR, 1 in partial remission (PR) that then converted to CR with incomplete recovery at 2 months, and 2 NR. No CD22-negative leukemia progression was observed.
Since our results with a long linker appeared inferior compared to the previously reported CART22 trial (short linker), we performed a direct comparison of the 2 different CAR22 constructs. In xenograft models, CART22SL significantly outperformed CART22LL (Fi 1 C) with improved overall survival. Moreover, CART22SL showed higher in vivo proliferation at day 17 (Fig 1 D). Mechanistically, intravital 2-photon imaging showed that CART22SL established more protracted T cell:leukemia interactions than did CART22LL, suggesting the establishment of productive synapses (Fig 1 E). Moreover, in vivo at 24 hrs higher T cell activation (CD69, PD-1) was observed in CART22SL from the BM of NALM-6-bearing mice.
Conclusions. Here we report the results of two pilot clinical trials evaluating the safety and feasibility of CART22 therapy for r/r B-ALL. Although feasible and with manageable toxicity CART22LL led to modest clinical responses. Preclinical evaluation allowed us to conclude that shortening the linker by 15 amino acids significantly increases the anti-leukemia activity of CART22, possibly by leading to more effective interactions between T cells and their targets. Finally, with the caveats of cross-trial comparison, our data suggest that xenograft models can predict the clinical efficacy of CART products and validate the use of in vivo models for lead candidate selection
Ruella: Novartis: Patents & Royalties, Research Funding. Maude: Novartis Pharmaceuticals: Consultancy, Other: Medical Advisory Boards. Engels: Novartis: Employment. Frey: Novartis: Research Funding. Lacey: Novartis: Research Funding; Genentech: Honoraria. Melenhorst: Novartis: Research Funding. Brogdon: Novartis: Employment. Young: Novartis: Research Funding. Porter: Incyte: Honoraria; Novartis: Honoraria, Patents & Royalties, Research Funding; Immunovative Therapies: Other: Member DSMB; Genentech/Roche: Employment, Other: Family member employment, stock ownship - family member; Servier: Honoraria, Other: Travel reimbursement. June: WIRB/Copernicus Group: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celldex: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immune Design: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Novartis: Patents & Royalties, Research Funding; Tmunity Therapeutics: Equity Ownership, Research Funding. Grupp: Jazz Pharmaceuticals: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Other: grant; University of Pennsylvania: Patents & Royalties; Adaptimmune: Consultancy. Gill: Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal